Abbott Announces CE Mark for i-STAT Alinity, a Pioneering, Handheld Blood Testing Platform
- Device is the first of Abbott's Alinity family of diagnostics instruments to launch
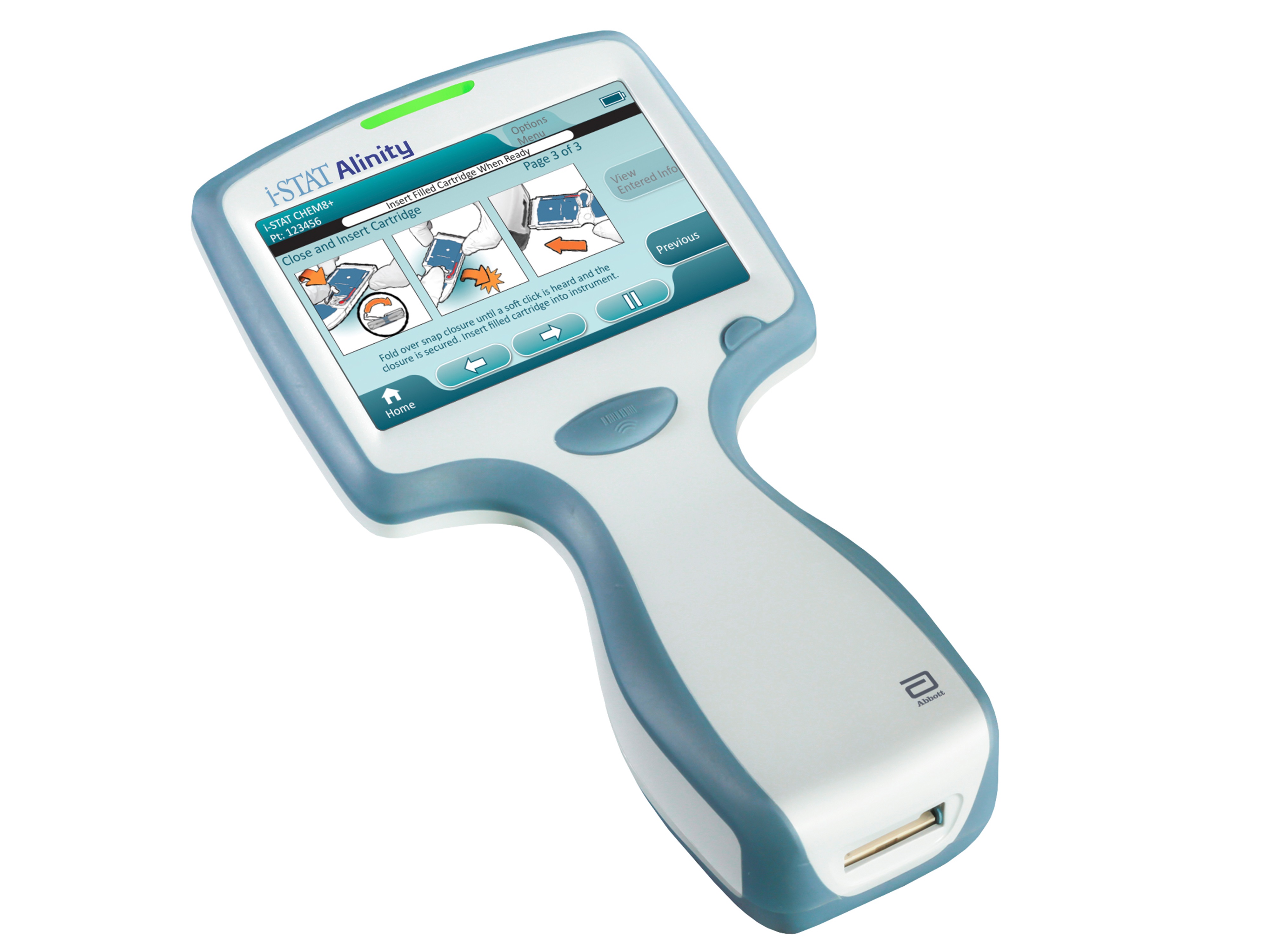
ABBOTT PARK, Ill., Nov. 30, 2016 /PRNewswire/ -- Abbott (NYSE: ABT) announced today its i-STAT® Alinity™ System, an innovative, handheld blood testing platform, received CE Mark and is now for sale in Europe and other countries that recognize CE Mark. The portable device can perform and analyze the largest menu of blood tests on a single device, ranging from blood chemistries to cardiac markers, using only two to three drops of a person's blood.1 Delivering results in two to 10 minutes, i-STAT Alinity equips healthcare professionals with the information they need to make fast and accurate medical decisions without ever leaving their patient's side. Its advanced connectivity features allow testing to be conducted virtually anywhere.
"As a global leader in point of care testing, Abbott's i-STAT Alinity builds on our rich diagnostics heritage to help healthcare providers deliver care when and where it's needed," said Sharon Bracken, vice president, Point of Care Diagnostics, Abbott. "Whether in emergency departments, critical care settings or rural areas, i-STAT Alinity allows clinicians to make timely and informed treatment decisions that help people get back to better health."
Globally, demand for healthcare is growing as people live longer, chronic diseases are more prevalent and people are more involved in decisions pertaining to their health. As more people access care, health systems, hospitals and other clinical care settings are challenged to keep pace and find resourceful, efficient and cost-effective ways to provide quality care.
To address these needs, Abbott developed i-STAT Alinity to be intelligent, intuitive and easy to use, empowering healthcare professionals to provide high-quality care for people across the globe. First-of-its kind, patented features include:
- Expansive testing menu capabilities to enable the most combinations of tests in a single cartridge and new types of tests in the future, including tests for brain injury, infectious disease and oncology
- Advanced quality control features designed with patient safety and regulatory compliance in the forefront
- Wireless or hard-wired connectivity so healthcare providers can manage diagnostic data and oversight with the broader healthcare system's network
- Cloud connectivity that uses a standard web browser, making it easy for health systems to customize multiple i-STAT Alinity instruments and provide seamless transmission of quality control data
- A large, bright, color touchscreen with graphics-driven, on-screen guidance and advanced light and sound notifications that alert clinicians with critical information
- An ergonomic design with easy-grip handling that is balanced, comfortable and secure
- Durable materials designed to prevent damage from drops and harsh cleaning substances commonly used in clinical settings
In today's pressure-packed healthcare environment, clinicians rely on easy-to-use testing technologies that do not require complex training. Innovative point of care testing tools like Abbott's i-STAT Alinity can help address this need and provide a simpler experience for those who use it, allowing them to accurately diagnose and treat patients faster and more efficiently.
DESIGNED WITH CUSTOMER-DRIVEN INSIGHTS
To better understand customer needs, Abbott sent cross-functional teams to visit administrators, clinicians and laboratory professionals to gain specific insights on the experiences and challenges healthcare providers face day-to-day with point of care testing.
"We spent countless hours doing on-the-ground research with doctors, nurses, lab directors, point of care coordinators and other customers from around the world. We asked about their pain points and listened to what they want and need for testing," said Matt Bates, divisional vice president, research and development, Point of Care Diagnostics, Abbott. "Using these customer insights, Abbott designed and built i-STAT Alinity for better access, efficiency and speed to improve clinical decision making and positively impact patients' lives."
i-STAT Alinity is the first of several next-generation instruments Abbott will launch as part of its harmonized family of Alinity systems across point of care, immunoassay, clinical chemistry, hematology, blood and plasma screening and molecular diagnostics. To learn more about Abbott's i-STAT Alinity, visit www.abbott.com/newsroom/news.html. Healthcare providers can find more information at https://www.abbottpointofcare.com/i-stat-alinity-ce.
About Abbott:
At Abbott, we're committed to helping you live your best possible life through the power of health. For more than 125 years, we've brought new products and technologies to the world—in nutrition, diagnostics, medical devices and branded generic pharmaceuticals—that create more possibilities for more people at all stages of life. Today, 74,000 of us are working to help people live not just longer, but better, in the more than 150 countries we serve.
Connect with us at www.abbott.com, on Facebook at www.facebook.com/Abbott and on Twitter @AbbottNews and @AbbottGlobal.
1 i-STAT Alinity is launching with a comprehensive test menu, including blood gases, chemistries, electrolytes and hematology. Commercial availability of cartridges may vary depending on geographic locations. Cardiac markers, coagulation and other tests will be available at a later date.
Logo - http://photos.prnewswire.com/prnh/20150928/271488LOGO
SOURCE Abbott
For further information: Abbott Media: Rachael Jarnagin, +1 (224) 668-6552 or Tracy Sorrentino, +1 (224) 668-0179; Abbott Financial: Mike Comilla, +1 (224) 668-1872
 BACK TO PRESS RELEASES
BACK TO PRESS RELEASES