



ABBOTT PARK, Ill., Jan. 30, 2015 /PRNewswire/ -- Two new intraocular lenses (IOLs) that restore vision to people with cataracts are now available in the United States. The new TECNIS® Multifocal IOLs, developed by Abbott (NYSE: ABT), provide people with cataracts options to have a full range of near, intermediate and distance vision, while customizing their treatment based on their personal vision needs and lifestyle.
Cataract surgery is one of the most common and safest procedures in the United States, with more than three million surgeries done each year. That number is expected to grow as the population gets older, and as some people choose to have cataract surgery sooner in life, given new technologies becoming available that can significantly improve vision.1
"Restoring or improving sight can make a huge impact on how people live their lives, which is why Abbott is continually investing in the research and development of advanced treatments to help people see their very best," said Murthy Simhambhatla, senior vice president, Medical Optics, Abbott. "We are pleased to offer the new TECNIS Multifocal IOLs for people with cataracts who want a full range of vision without having to wear eyeglasses, and who also want high-quality vision at a specific distance that is suited to their lifestyle."
An IOL is an artificial lens placed in a person's eye to restore vision after a cataract has been removed. While traditional monofocal IOLs are designed to restore far or distance vision, multifocal IOLs provide quality vision at multiple distances and reduce or eliminate the need for glasses.
The new lenses approved by the U.S. Food and Drug Administration are known as TECNIS Multifocal +2.75D and +3.25D IOLs. Along with the available TECNIS Multifocal +4.0D IOL, all of the TECNIS Multifocal IOLs provide excellent vision at near, intermediate and far distances, while each "lens power" version provides enhanced vision at a distinct distance.

The TECNIS Multifocal +4.0D is suited for people favoring near vision-related activities, such as reading a book. The TECNIS Multifocal +3.25D IOL is suited for people who favor activities at longer reading distances, such as reading a hand-held device like a tablet. The TECNIS Multifocal +2.75D IOL is suited for people who favor activities done with intermediate vision, such as reading labels while grocery shopping.
"My patients are extremely satisfied with the new TECNIS Multifocal lenses," said Dr. Kerry Assil, medical director of the Assil Eye Institute in Los Angeles. "Many of them are able to read easily, not only up close, but at intermediate distance as well, and in all lighting conditions. This improved vision following cataract surgery helps people live the active lives they want."
The TECNIS Multifocal IOLs have been well-studied. The FDA approval of the new TECNIS Multifocal +2.75D and +3.25D IOLS is based on a study of 445 people with cataracts. The results showed:
- More than 80 percent of people reported being able to function comfortably without glasses at all distances
- 93 percent of patients reported they would have the same IOL implanted again
- Patients had a mean visual acuity of 20/25 in both eyes at near distances (uncorrected, or without glasses or contact lenses)
- Patients had a mean visual acuity of 20/20 in both eyes at far distances (uncorrected)
- Good vision was maintained in low lighting conditions
About Abbott:
Abbott is a global healthcare company devoted to improving life through the development of products and technologies that span the breadth of healthcare. With a portfolio of leading, science-based offerings in diagnostics, medical devices, nutritionals and branded generic pharmaceuticals, Abbott serves people in more than 150 countries and employs approximately 77,000 people.
Visit Abbott at www.abbott.com and connect with us on Twitter at @AbbottNews.
Important indications and safety information - TECNIS Multifocal 1-Piece IOLs (for prescription only)
ATTENTION: Please see Directions for Use for a complete list of important indications, warnings, precautions, and adverse events.
INDICATIONS: The TECNIS Multifocal 1-Piece intraocular lenses, Models ZKB00 (+2.75 D) and ZLB00 (+3.25 D), are indicated for primary implantation for the visual correction of aphakia in adult patients with and without presbyopia in whom a cataractous lens has been removed by phacoemulsification and who desire near, intermediate, and distance vision with increased spectacle independence. The intraocular lenses are intended to be placed in the capsular bag.
IMPORTANT SAFETY INFORMATION: Patients may experience possible contrast sensitivity reduction and increases in visual disturbances that may affect their ability to drive at night or in poor visibility conditions. Healthcare professionals should weigh the potential risk/benefit ratio for patients with conditions that could be exacerbated or may interfere with diagnosis or treatment. Secondary glaucoma has been reported occasionally in patients with previously controlled glaucoma who received lens implants. Multifocal IOL implants may be inadvisable in patients with conditions such macular degeneration, retinal pigment epithelium changes, and glaucoma. In rare cases, severe incidents of halos and/or glare may result in the multifocal IOL being explanted and replaced with another type of IOL.
1. 2014 Comprehensive Report on the Global IOL Market, Market Scope®, LLC

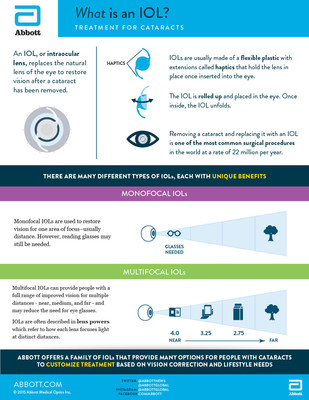
Photo - http://photos.prnewswire.com/prnh/20150129/172399
Photo - http://photos.prnewswire.com/prnh/20150129/172400-INFO
Photo - http://photos.prnewswire.com/prnh/20150129/172401-INFO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/abbott-launches-new-options-for-people-with-cataracts-in-the-united-states-300028263.html
SOURCE Abbott
 BACK TO PRESS RELEASES
BACK TO PRESS RELEASES