Month Year
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
Month Year
| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
-
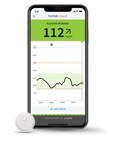
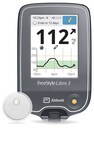
May 11, 2023The Eterna™ spinal cord stimulation (SCS) system is FDA approved for wider access to scans, allowing for more options for people in need of imagingRadiologists can use normal operating mode on the Eterna SCS system, permitting for higher quality scan images1,2